LAB 4: SOURCES OF CONTAMINATION AND INFECTION
Introduction
Microbiological contamination refers to the non-intended or accidental
introduction of infectious material like bacteria, yeast, mold, fungi, virus,
prions, protozoa or their toxins and by-products. Major contamination sources are water,
air, dust, equipment, sewage, insects, rodents, and employees. Contamination of
raw materials can also occur from the soil, sewage, live animals, external
surface, and the internal organs of meat animals. Airborne
microorganisms are biological airborne contaminants (also known as bio-aerosols)
like bacteria, viruses or fungi as well as airborne toxins passed from one
victim to the next through the air, without physical contact, causing
irritation at the very least. So, microbiologists need to be
careful while dealing with airborne microorganisms which have the potential for
contamination. The risk of getting contamination can be avoided by careful
observation of simple precautions.
The human body is made up of about 10 trillion cells, but hosts
100 trillion more. The vast majority of cells living on and in the body are
bacteria and other microbes. For example, normal human skin is colonized by bacteria, with total
aerobic bacterial counts ranging from more than 1 × 106 CFU/cm2 on the scalp, 5 × 105 CFU/cm2 in the axilla and a ranged from 3.9 ×
104 to 4.6 × 106 CFU/cm2
on our hands. Total
Viable Count (TVC) gives a
quantitative idea about the presence of microorganisms such as bacteria, yeast
and mold in a sample. To be specific, the count actually represents the number
of colony forming units (CFU) per g (or per ml) of the sample.
A TVC is achieved by plating dilutions of the culture until 30-300 colonies
exist on a single plate.
Microorganisms
can enter the body through the four sites which are respiratory tract (mouth
and nose), gastrointestinal tract (mouth oral cavity) e.g. Vibrio
cholerae which causes cholera, urogenital tract e.g. Escherichia
coli which causes
cystitis and lastly, breaks in the skin surface e.g. Clostridium tetani which
causes tetanus. The skin and mucous
membranes always harbor a variety of microorganisms that can be arranged into
two groups which are the resident
microorganisms and transient microorganisms.
Resident microorganisms,
also known as commensal microorganisms are microorganisms that resides in the
body such as a bacteria or yeast but that doesn’t cause harm or negatively
impact health. Resident microorganisms typically colonize the surface of the
skin, mucous membranes, digestive tract, upper respiratory system and distal
portion of the urogenital system. The
factors that make pathogenesis is more likely include a breach in the
mechanical skin barrier due to injury, a skin disease or an invasive medical
device, immune suppressant medication, immunocompromise due to cancer or HIV,
extremes of age and individual genetic factors.
Transient
microorganisms consists of non-pathogenic or potentially pathogenic
microorganisms derived from environment that inhabit the skin or upper
respiratory tract. Although they may attempt to colonize the same areas of the
body as do resident microorganisms, but they are unable to remain in the body
for extended periods of time due to difficulty competing with established
resident microbes, elimination by the body’s immune system and physical or
chemical changes within the body that discourage the growth of transient
microbes.
Infection is
the invasion and multiplication of pathogenic microorganisms in the body. When
we are infected by pathogens we become sick, which means that our bodies stop
functioning properly. Infectious agents, such as bacteria, a virus, fungi or
protozoa cause communicable diseases which can be spread from one person to
another. Infection caused by pathogens that take advantage
of an opportunity not normally available is known as opportunistic infection.
This type of infection normally happened to a host with weakened immune system, an altered microbiota (such as a disrupted gut flora), or breached integumentary barriers.
As the bacteria consume the nutrients, they begin to grow and multiply.
This generates thousands to millions to billions of cells that begin to pile
up, becoming visible to the naked eye. This pile of cells originates from one
cell and is called a bacterial colony. Each species of
bacteria produces a colony that looks different from the colonies produced by
other species of bacteria. Examination of the form and structure of bacterial
colonies is termed colony morphology and is one of the first steps in
characterizing and identifying a bacterial culture. The basic characteristics
of colony morphology that are typically evaluated are the form, size, shape, texture,
elevation, color and surface.
Figure
1: Characteristics of colony morphology
The pour plate method involved molten application of all
of the agar subsequently found within a plate
or instead application of just a thin, top layer of agar as seen with the soft agar overlay technique. Note that
it is important in overlay methods to employ agar that is sufficiently warm but
not too warm. A typically temperature employed is 45°C. This
prevents premature solidification of the agar while at the same time, ideally,
does not excessively overheat organisms. While
doing pour plate method, one must ensure no molten agar is splashed over the
side or lid of the plate. An inoculated plate must be incubated in an inverted
position to prevent condensation from falling onto the surface of the agar and
interfering with discrete colony formation.
Figure 2: Pour plate
method
Streak
plate technique is used for the isolation into pure culture of the organisms (mostly
bacteria), from mixed population. The inoculum is streaked over the agar
surface in such a way that it “thins out” the bacteria. Some individual
bacterial cells are separated and well spaced from each other. As the original
sample is diluted by streaking it over successive quadrants, the number of
organisms decreases. Usually by the third or fourth quadrant only a few
organisms are transferred which will give discrete colony forming units
(CFU). When these lone bacterial cells divide and give rise to thousands and
thousands of new bacterial cells, an isolated colony is
formed. Pure cultures can be obtained by picking well isolated colonies and
re-streaking these on fresh agar plates. While
streaking the inoculum, make sure surface of the plate is free
of droplets of condensed moisture. Then, use only a small amount of inoculums
and streak lightly so that one does not gouge the agar.
Figure 3: Streak plate method
Figure 4: Results of streak plate method
Objective
To
determine the microorganisms in the air and from the healthy humans.
Materials and reagents
Molten
commercial nutrient agar
Molten self-made
nutrient agar
Sterile
water
Sterile petri dishes
Sterile
clinical swab
Pipette
and tips
Bunsen
burner
70% ethanol
1. The
work bench is sterilized with 70% ethanol.
2. Sources
of contamination are labelled at the bottom of sterile petri dishes.
3. The
cap of the Scott bottle which contains commercial nutrient agar is removed. The
neck of the Scott bottle is flame-sterilized using Bunsen burner.
4. The
lid of the sterile petri dish is opened slightly, then the commercial nutrient
agar is poured slowly into the sterile petri dish.
5. The
lid of the sterile petri dish is replaced immediately.
6. The
cap and the neck of Scott bottle is flame-sterilized again and is recapped.
7. The
sterile petri dish is left to settle down until the agar is set.
8. Step
3 to step 7 are repeated using molten self-made nutrient agar.
Sources
of contamination:
A. Air
1. The
lid is removed from the plate and is left to rest on the side of the plate,
facing down. The plate is left to be exposed for about 5 minutes. The lid is
replaced.
2. The
dish is inverted and is incubated at 37°C for 48 hours.
B. Hands
1. Hand
is washed using sterile water. Soap is not allowed to use.
2. An
automatic pipette is used to transfer 1ml of wash water to the petri dish.
3. The
molten nutrient agar is added to the petri dish.
4. The
lid of petri dish is replaced and the dish is gently rotated by doing 5 times
figure of ‘8’ motion until the wash water is thoroughly mixed with the molten
agar. The agar is not allowed to contact with the lid of the dish.
5. The
dish is inverted and is incubated at 37°C for 48 hours after the agar has set.
C. Ear
1. A
sterile swab moistened with sterile isotonic solution is rubbed into the ear of
the subject using extreme care.
2. The
lid is removed. The swab is used to inoculate the labelled plate. The inoculum
is distributed in streak method. The lid is replaced.
3. The
dish is inverted and is incubated at 37°C for 48 hours.
D. Normal Breathing
1. The
lid is removed. The plate is held about 15cm from the mouth and breathe
normally but directly onto the plate for one minute. The lid is replaced.
2. The
dish is inverted and is incubated at 37°C for 48 hours.
E. Violent Coughing
1. The
lid is removed. The plate is held about 15cm from the mouth and cough violently
onto the agar. The lid is replaced.
2. The
dish is inverted and is incubated at 37°C for 48 hours.
Figure
5: All petri dishes are inverted and are incubated at 37°C for 48 hours.
Result and observation
1. 1. Air
Figure
6: Sample from the air as the source for contamination.
(Left:
Commercial agar, Right: Self-made nutrient broth agar)
2. Hand
Figure 7: Sample from
the hand as the source for contamination.
(Left:
Commercial agar, Right: Self-made nutrient broth agar)
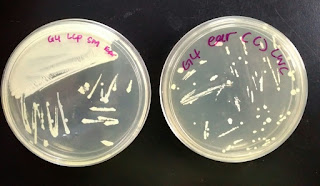
3. Ear
(Left: Self-made nutrient broth agar, Right: Commercial agar)
4. Normal Breathing
Figure
9: Sample from the normal breathing as the source for contamination.
(Left: Commercial agar, Right: Self-made
nutrient broth agar)
5. Violent Coughing
Figure 10: Sample from the violent coughing as the source for contamination.
(Left:
Self-made nutrient broth agar, Right: Commercial agar)
Based
on the observation (shown from Figure 6 to 10), the forms, elevation, surfaces,
textures, margins and colors of the microorganism colonies are tabulated below
(shown in Table 1):
Table
1: The forms, elevation, surfaces, textures, margins and colors of the
microorganism colonies in self-made nutrient broth agar and commercial agar.
Type of Agar
|
Sample
|
Color
|
Form
|
Elevation
|
Surface
|
Texture
|
Margin
|
Self-made nutrient broth agar
|
Air
|
White,
Yellow, Pale yellow.
|
Circular.
|
Flat.
|
Shiny.
|
Mucoid.
|
Entire.
|
Hand
|
White,
Yellow, Pale yellow.
|
Circular.
|
Flat.
|
Rough.
|
Dry.
|
Entire.
|
|
Ear
|
White,
Yellow, Pale.
|
Irregular,
Circular.
|
Flat.
|
Rough.
|
Mucoid.
|
Lobate.
|
|
Normal
Breathing
|
Semi-transparent,
White, Pale Yellow.
|
Rhizoid,
Circular.
|
Raised.
|
Rough.
|
Viscous.
|
Lobate.
|
|
Violent
Coughing
|
Semi-transparent,
White.
|
Irregular.
|
Flat.
|
Rough.
|
Dry.
|
Entire.
|
|
Commercial Agar
|
Air
|
Semi-transparent,
White, Pale Yellow, Yellow.
|
Rhizoid,
Circular.
|
Flat.
|
Rough.
|
Moist.
|
Lobate.
|
Hand
|
White,
Yellow, Pale yellow.
|
Circular,
Irregular.
|
Raised.
|
Shiny.
|
Mucoid.
|
Lobate.
|
|
Ear
|
White,
Yellow, Pale yellow.
|
Circular,
Irregular.
|
Flat.
|
Shiny.
|
Dry.
|
Entire.
|
|
Normal
Breathing
|
Semi-transparent,
Pale Yellow.
|
Rhizoid,
Circular.
|
Concave.
|
Shiny.
|
Moist.
|
Lobate.
|
|
Violent
Coughing
|
Yellow,
White
|
Circular.
|
Concave.
|
Shiny.
|
Viscous.
|
Entire.
|
Discussion
Microorganisms
will grow in a very high rate under favorable environmental conditions and vital
nutrients required. As the microorganisms grow, colonies are formed. Different
microorganisms produce colonies with different appearances called “colony
morphology”. There are seven aspects in “colony morphology” such as form,
elevation, size, surface, texture, color and margin.
A)
Air
The
culture media are exposed to the air outside the laboratory. The air always has
many types of microorganisms floating around. Thus, the observation from the
culture media shows that there are different microorganisms growing on the
agar. Typically, Staphylococci, Bacillus and Clostridium can be found in the
air.
B)
Hand
The
similarities between both culture media is that the number of colonies formed
are high. This indicates that our hands contain lots of microorganisms as we
make contact with objects contaminated by Streptococcus, Mycobacteria, Haemophilus and etc.
C)
Ears
Streak
method is used to transfer the microorganisms collected on the ear swab from
our ears to the culture media. From the observation, large number of
microorganisms are found on the agar. This is because our ears are exposed to
the air. Microorganisms that usually can be found on the ears are Candida albican, Corynebacterium,
Pseudomonas aeruginosa and Staphylococcus aureus.
D)
Normal breathing
The
presence of colonies in the culture media shows that microorganisms can also be
found in the mouth and nasal cavity. Compared to other culture media, there is
relatively low number of colonies from the contamination of normal breathing.
Examples of microorganisms are Streptococcus, Haemophilus, Micrococcus and
Corynebacterium
diphtheriae.
E)
Violent coughing
The
observations are similar to that from the normal breathing due to same sources
of contamination, that is mouth and nasal cavity. However, coughing forces more
air out of the mouth and therefore slightly more colonies are found in the
culture media. Examples of microorganisms are Streptococcus pneumoniae,
Streptococcus salivarius and
Staphylococcus epidermidis.
Conclusion
From
this experiment, we can conclude that microorganisms are able to grow provided
that the environmental conditions introduced to them are suitable. Both the
commercial agar and self-made nutrient agar provide favorable environment and
sufficient nutrients for microorganisms to grow. Then, we know that more
microorganisms are found outside the body like hands and ears than inside the
body that are obtained from normal breathing and violent coughing. Furthermore,
we are able to identify microorganisms according to the colony morphology. For
example, bacteria colony typically has a smooth surface. In contrast, fungi
colony usually has a rough surface.
References
https://www.boundless.com/microbiology/textbooks/boundless-microbiology-textbook/culturing-microorganisms-6/culturing-bacteria-58/aseptic-technique-dilution-streaking-and-spread-plates-367-7652/
http://www.microbiologyonline.org.uk/about-microbiology/microbes-and-the-human-body/microbes-and-disease
http://microbeonline.com/colony-morphology-bacteria-describe-bacterial-colonies/
http://microbeonline.com/streak-plate-method-principle-purpose-procedure-results/
http://study.com/academy/lesson/bacterial-colony-morphology-characteristics-definition.html